New Project 4 Publications Evaluate Cost-Efficient Water Treatments Using Banana Peels
Two new publications by Project 4 researchers present experiments that used banana peel-derived cathodes to test cost-effective water treatments that remove organic contaminants. The findings from these studies can be used to inform large-scale, practical application of treatments in water systems, including Puerto Rico’s Karst system.
One paper led by PhD candidate Jong-Gook Kim describes how researchers established a banana peel-derived biochar (BP-BC) cathode packed in a stainless steel mesh and used it in a continuous flow reactor to degrade ibuprofen in water. One goal of the study was to see how a carbon enriched biochar, which is an inexpensive and environmentally friendly catalyst for removing contaminants from water, reacts when used in electrochemical advanced oxidation processes (EAOPs). The ibuprofen acted as the model contaminant, and the water used was simulated Puerto Rico karst groundwater so that researchers could understand how the process would work in the system that PROTECT participants use.
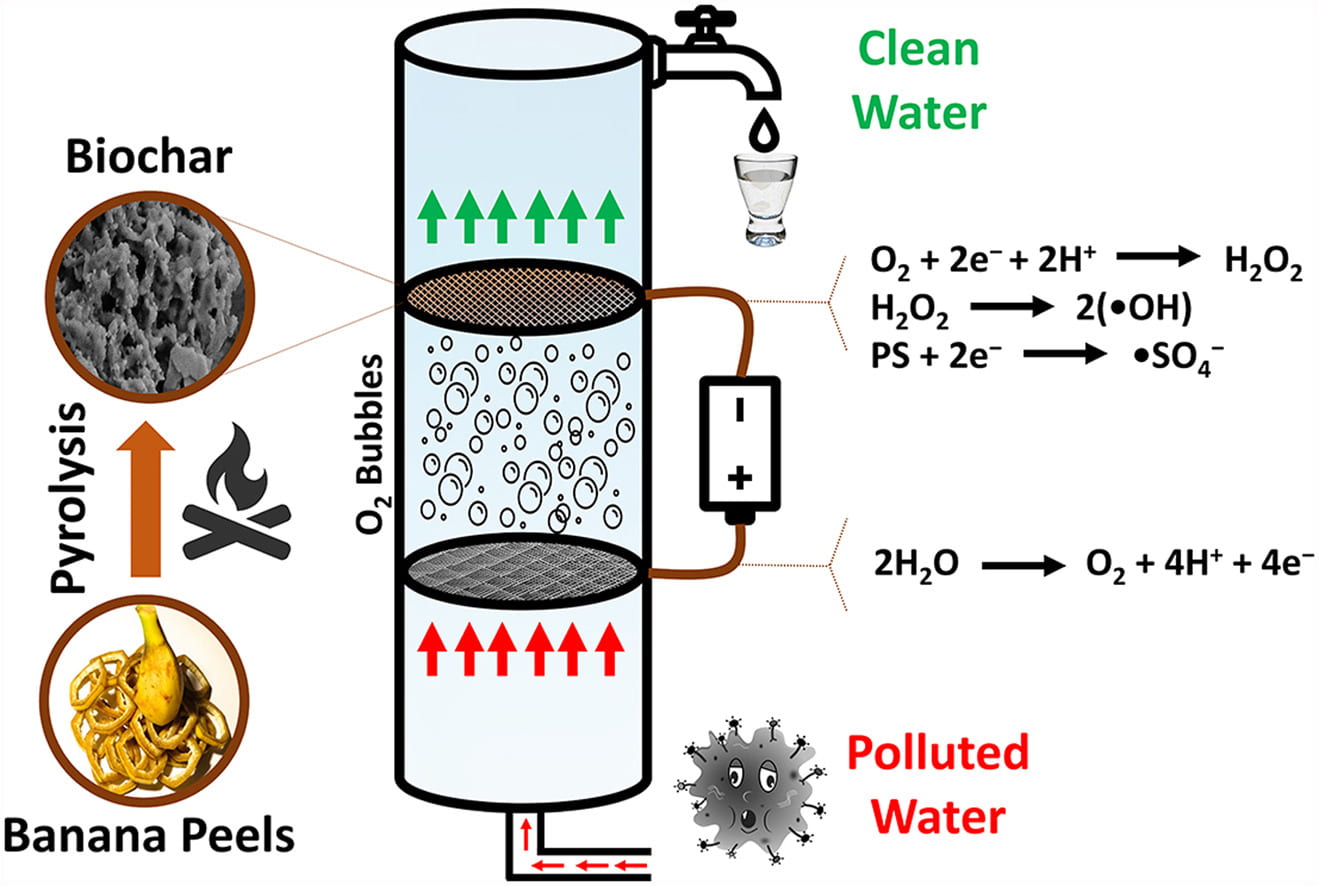
Graphical abstract for Kim’s paper
In an EAOP set up in this study, the stainless-steel mesh distributes the current to the banana peel cathode, which generates hydrogen peroxide (H2O2) through a 2-electron oxygen reduction reaction (2e-ORR). This porous surface of biochar enables efficient decomposition of H2O2 to generate •OH, which degrades ibuprofen, the model contaminant. The tests performed showed limited H2O2 generation, which resulted in just 40% ibuprofen degradation. To enhance this degradation, researchers added persulfate (PS) to the system. Through tests of different PS concentrations, they found that the presence of 10mM PS led to 100% ibuprofen degradation, showing that more affordable cathodes can be used in EAOPs and remove organic contaminants with the help of other chemicals.
The findings that the presence of PS enables complete ibuprofen removal are important. However, adding PS is not desirable for large-scale applications of water treatments. This was addressed in another study that used the banana peel-derived biochar encased in a stainless-steel mesh (BBSS). In this study, researchers evaluated how polarity reversal, which changes direction of electrical current flow, could activate the BBSS cathode via anodization and generate oxygen functionalities for the 2e-ORR. Researchers pre-anodized the banana peel-derived cathode to improve the cathode’s corrosion and abrasion resistance via polarity reversal at different current densities and time durations. The electrocatalytic performance of the cathodes was evaluated with two reference anodes: a stainless-steel mesh anode, and a nickel foam supported manganese-doped tin oxide (Mn-SnO2@NF) anode for oxygen evolution reaction. Bromophenol blue and Congo red dyes acted as the model contaminants. In the experiments, the Mn-SnO2@NF anode in combination with the pre-anodized BBSS cathode generated H2O2 at a rate of 9.4 mg/L, and resulted in 87.44% degradation of the blue dye and 86.63% degradation of the red dye. In comparison, the stainless-steel mesh anode generated H2O2 at a rate of 7.1 mg/L. Although the Mn-SnO2@NF anode enabled higher H2O2 generation, researchers determined that the stainless-steel mesh also worked well and would be the most cost-effective and practical anode to use in large scale applications.
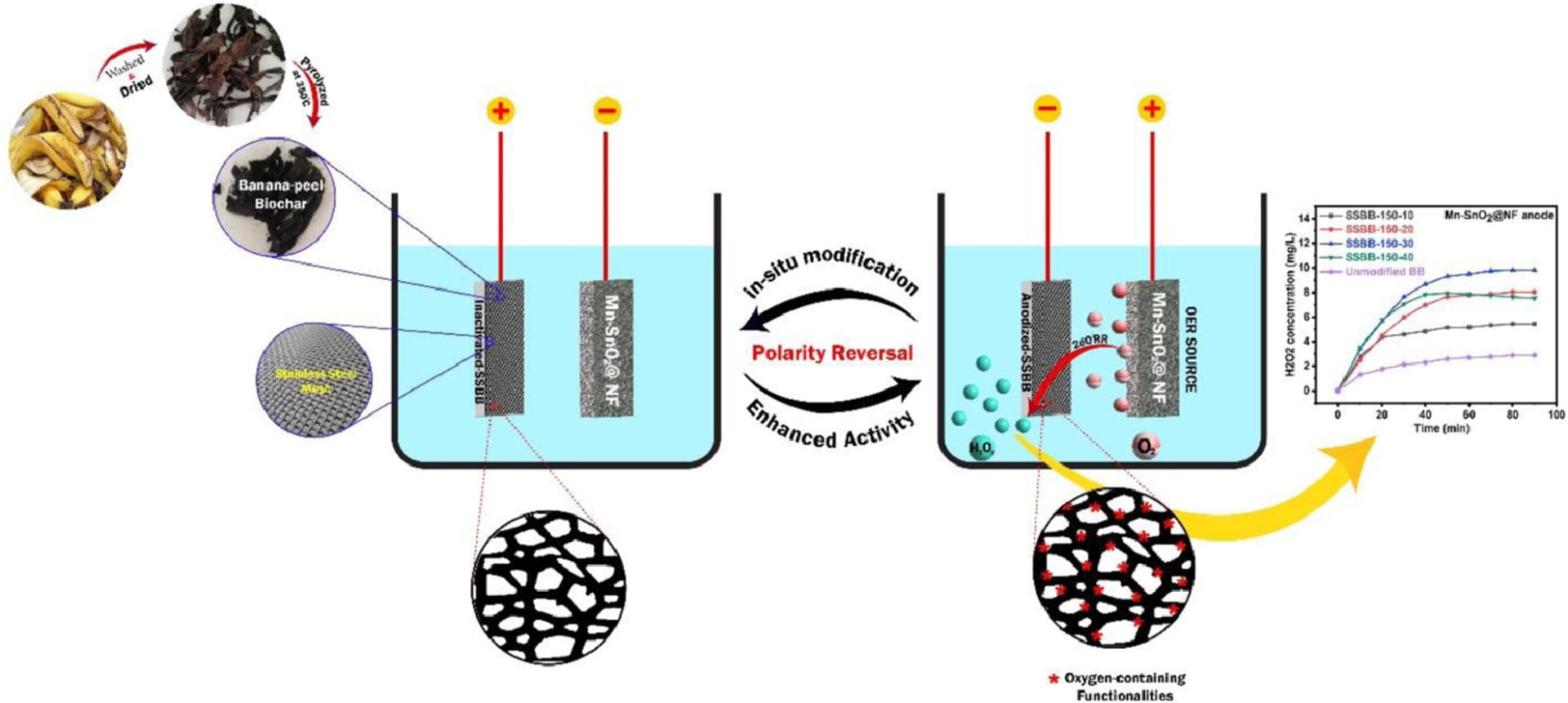
Graphical abstract for the second paper
Findings presented in these papers demonstrate that affordable water treatment methods can effectively generate H2O2 to remove organic contaminants from groundwater. Further studies can use this knowledge to develop practical, large-scale water treatments that can be used in Puerto Rico’s water systems.